
What is the Difference Between Positive and Negative Ion? Negative ions can be atoms or moieties of molecules. These anions form when a neutral chemical species gains an electron from its chemical structure. Negative ions are chemical species which carries a negative electrical charge. Moreover, these ions are in several forms as monoatomic ions, diatomic ions or polyatomic ions according to the number of atoms present in the ionic species.Įx: K +, Na +, NH 4 +, etc. This gives a net positive charge to the atom or molecule.
Therefore, when this atom or molecule loses an electron, there is an extra proton which has a positive charge. These species have protons and electrons in equal amounts in order to neutralize the charges. Positive ions can be atoms or moieties of molecules. These cations form when a neutral chemical species loses an electron from its chemical structure.
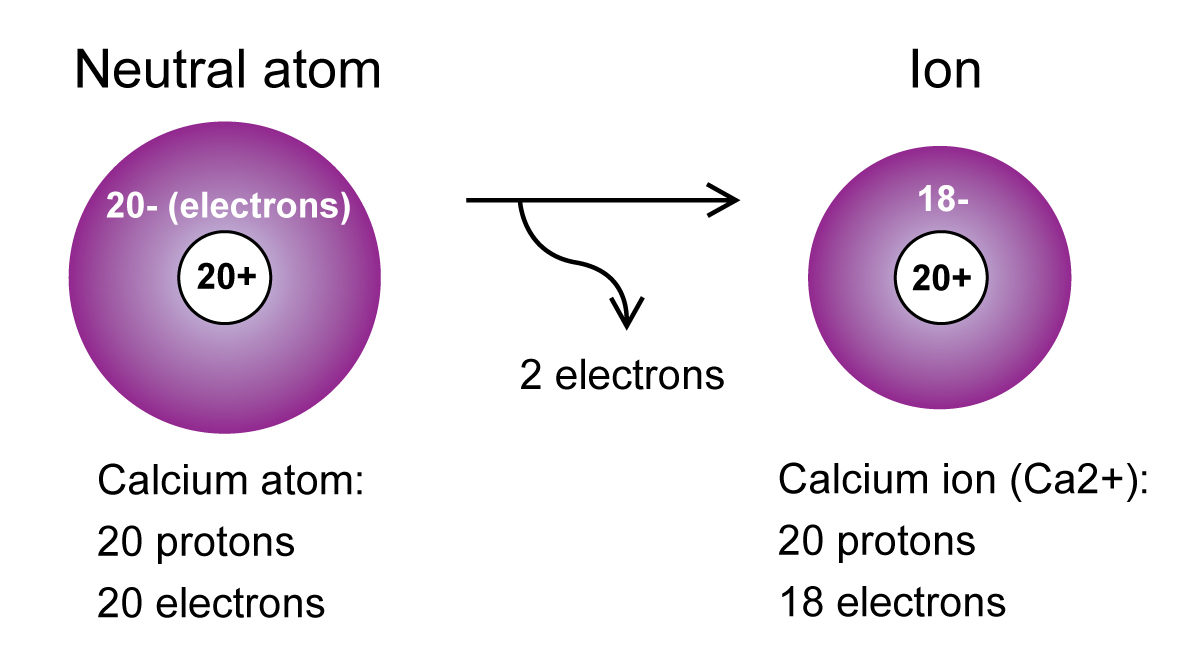
Positive ions are chemical species that carries a positive electrical charge. Side by Side Comparison – Positive vs Negative Ion in Tabular Form Hence, there are two forms of ions as positive ions and negative ions. This charge can be either positive or negative.
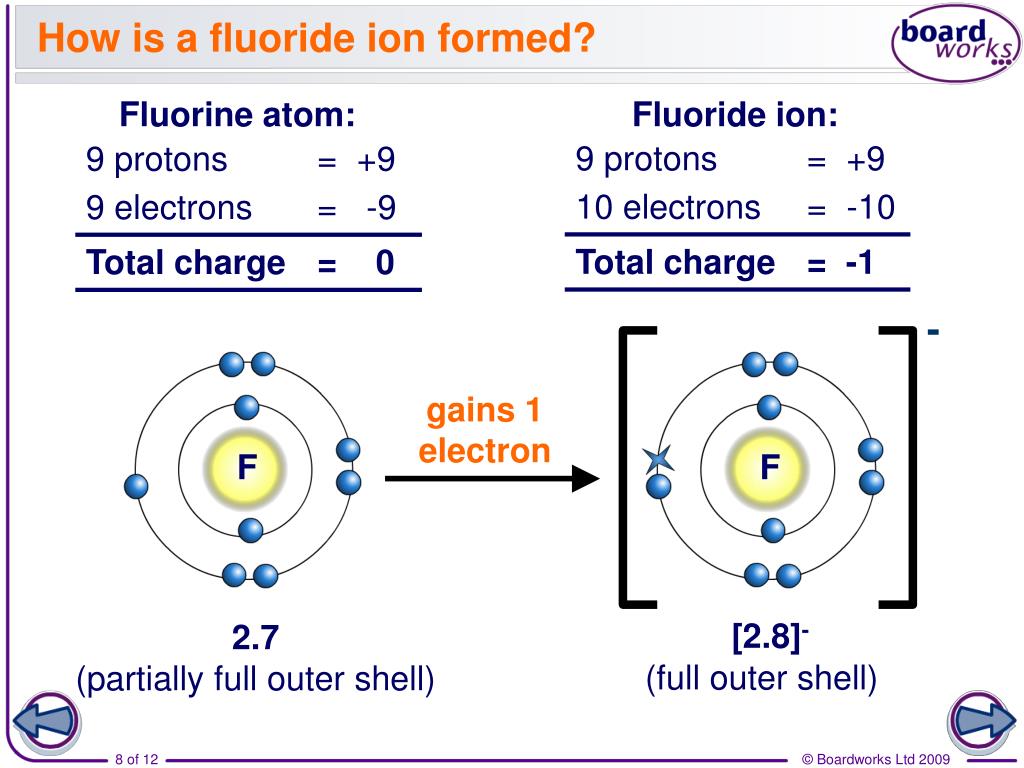
Therefore, these species carry an electrical charge. Ions are chemical species that derive from either the electron loss or gain. The key difference between positive and negative ion is that the positive ion carries a positive electrical charge whereas the negative ion carries a negative electrical charge.


 0 kommentar(er)
0 kommentar(er)
